Background:
Thrombopoietin-receptor agonists (TPO-RAs) are used in the treatment of chronic immune thrombocytopenia (ITP), a disorder characterized by prolonged low platelet counts (PCs) that pose a risk of serious bleeding events. The TPO-RAs eltrombopag (ELT) and romiplostim (ROMI) have been available for more than 10 years. ELT is an oral medication with food restrictions and carries a boxed warning for hepatoxicity, requiring hepatic laboratory monitoring and a potential need for statin dosing adjustments. ROMI, an injectable, is typically administered in a health care practitioner's office on a weekly basis without food restrictions. Avatrombopag (AVA), an oral medication taken with food, is the most recently approved TPO-RA for the treatment of chronic ITP (2019 by FDA and 2021 by EMA).
Hence, the real-world evidence on AVA treatment and its clinical effectiveness in broader and more diverse ITP patient populations have been limited, and additional studies are warranted. REAL-AVA 2.0 is a retrospective chart review which will gather a substantially greater amount of platelet counts per patient than either a claims database review or an electronic medical record review. More granular efficacy data with more patients, more platelet counts per patient, and in a broader population will be used to better assess the clinical effectiveness of AVA in the real-world.
This study will also describe the demographic and clinical characteristics of adult patients with primary ITP treated with AVA in the United States (US), and assess treatment patterns and clinical outcomes of these patients.
Methods:
This retrospective, observational medical chart review study will include patients treated in the US with AVA in a non-clinical trial setting from approximately 20 centers (both academic and community). The study aims to include approximately 200 ITP patients (at least 25 with an ITP disease duration < 12 months) treated with at least one dose of AVA between July 2019 and March 2023. The date the patient initiated treatment with AVA is defined as the index date. The period from the index date through the study end date, end of data availability for the patient or date of death, whichever occurs first, is defined as the post-index period.
Results:
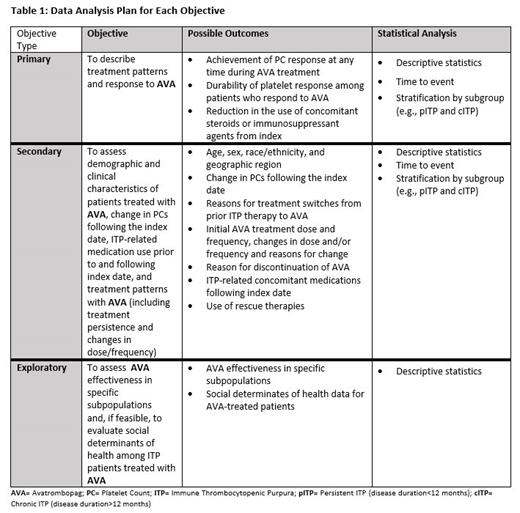
The primary objective of the study is to describe treatment patterns and response to AVA. Primary outcome measures to be assessed include: achievement of meaningful PC thresholds (PC ≥ 30 X10 9/µL and ≥ 50 X10 9/µL) at any time during AVA treatment, time to achieve an initial platelet response with AVA based on achieving meaningful PC thresholds (PC ≥ 30X10 9/µL and ≥ 50 X10 9/µL) after AVA index date and before AVA discontinuation, durability of platelet response among patients who respond to AVA, and reduction in the use of concomitant steroids or immunosuppressant agents from the index date. The secondary objectives include: assessment of the demographic and clinical characteristics of patients treated with AVA, assessment of changes in PCs following the index date, ITP-related medication use prior to and following the index date, and treatment patterns with AVA (including treatment persistence and changes in dose/frequency). The exploratory objectives include assessment of AVA effectiveness in specific subpopulations (e.g. prior number of ITP treatments, persistent vs chronic ITP, switch from particular ITP treatments, gender, race, age) and, if feasible, to evaluate social determinants of health among ITP patients treated with AVA (including assessment of treatment response among those subpopulations of interest) [ Table 1].
Summary/Conclusions:
The study aims to fill current gaps in knowledge by assessing treatment patterns and outcomes, clinical, and demographic characteristics of patients treated with AVA in a real-world clinical setting in the US. We look forward to presenting this data at upcoming scientific meetings.
Disclosures
Nagalla:Alexion Pharmaceuticals, Sanofi, Agios, Apellis, Rigel, Sobi, Pharmacosmos: Consultancy; Alexion, Sanofi, Agios, Apellis, Rigel, Sobi: Speakers Bureau. Chaturvedi:Alexion: Consultancy, Other: Advisory board participation; Takeda: Other: Advisory board participation; Sanofi Genzyme: Consultancy; Sobi: Honoraria; Sanofi: Other: Advisory board participation. Levy:Abbvie: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; AZ: Consultancy, Honoraria, Speakers Bureau; Beigene: Consultancy, Honoraria, Speakers Bureau; Bristol Meyer Squibb: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Genmab: Consultancy, Honoraria, Speakers Bureau; Jazz: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Speakers Bureau; Morphosys: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seagen: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Sobi: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Sellas: Membership on an entity's Board of Directors or advisory committees. Kolodny:Sobi, Inc.: Current Employment. Oladapo:Sobi, Inc: Current Employment. Bernheisel:Sobi, Inc.: Current Employment. Jamieson:Sobi, Inc.: Current Employment. Goldschmidt:Sobi, Inc.: Consultancy. Swallow:Sobi, Inc.: Consultancy. Greatsinger:Sobi, Inc.: Consultancy. Vredenburg:Sobi, Inc.: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal